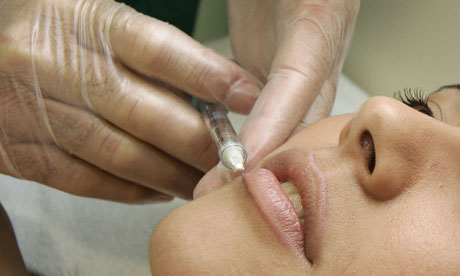
Thousands of patients who have received anti-ageing injections could be at risk of serious complications, senior doctors have warned, raising fears about the safety of non-surgical cosmetic procedures.
Doctors said the "facial fillers" can cause infections and deformities and are often injected into patients by unqualified practitioners.
Patients who had fillers up to 10 years ago are now coming forward with complaints of discomfort and other complications.
The British Association of Dermatologists has called for the MHRA (the UK's Medicines and Healthcare Products Regulatory Agency) to reclassify fillers as medicines, rather than the current "devices", and regulate them more tightly.
The warning came as European health authorities issued widely different recommendations in dealing with the French-made Poly Implant Prothese (PIP) breast implants.
Germany's Federal Institute for Drugs and Medical Devices said that it was recommending the removal of PIP breast implants "as a precautionary measure", while the Czech health ministry said about 2,000 Czech women with potentially faulty implants should have them removed.
The health secretary, Andrew Lansley, said the UK had found no link between the implants and cancer. But he added that the non-medical grade silicone used by the now defunct PIP company "should not have been implanted in women in the first place", and offered to provide examinations and possible removals in instances where doctors said they were necessary.
The Times reports that anti-ageing fillers were injected into thousands of people each year and some clinics were using a filler "with a 5% complication rate".
Bio-Alcamid, a wrinkle-smoothing gel which is not approved for use in the US, can "migrate" from where it is originally injected and cause lumps under the skin, doctors said.
Fazel Fatah, president of the British Association of Aesthetic Plastic Surgeons, told the Times: "Anyone who has a permanent filler must be made aware that in the long term they can have problems, including infections or reactions to the materials."
Dr Sean Cummings, of the Freedom Health Clinic in Harley Street, said most practitioners, who do not have to be doctors, learned to use Bio-Alcamid during a single afternoon observation session at a lecture theatre in London.
He told the paper: "There have been polarised views. Some people say it is safe enough to use and I have had some good results. I have seen complications in the range of 4.5-5%, which is not safe. I have stopped using it."
Polymekon, the Italy-based manufacturer of Bio-Alcamid, has said that the gel itself had a 1% rate of complication and did not migrate. It claimed that most problems were likely to have involved poor administration or subsequent procedures introducing bacteria into the implants.
The MHRA said it had no specific concerns over the product, providing it was used according to the manufacturer's instructions.

