Labour is urging the government to give a much clearer lead to more than 40,000 women in the UK possibly affected by the scandal over faulty breast implants.
The shadow health secretary, Andy Burnham, called for a plan to be agreed with the private cosmetic surgery industry spelling out what should be done for women who have substandard breast implants filled with industrial-grade silicone gel – and who should pay for it.
Women were being made very anxious, he said, and clarity was needed to reassure them. "Mixed messages from the department have not helped, and families affected are looking for a stronger response from the government.
"Patients' stories are emerging suggesting that the response from some private providers has been completely inadequate. For instance, it is simply unacceptable for women whose implants have ruptured, causing pain and discomfort, to be referred back to the NHS. They need urgent help.
"What the thousands of women affected need is for the government to agree a clear protocol with the private cosmetic surgery industry, setting out the support that they will provide."
Labour's intervention came amid warnings from experts that anti-ageing injections will be the next scandal in the cosmetic surgery industry.
Sally Taber, director of Independent Healthcare Advisory Services, the cosmetic surgery industry body, said that the injections were often performed by beauty therapists with just a few hours' training. There are 160 injectable fillers certified for sale in the UK that can be bought and used by anyone, leaving patients at risk of poor-quality products.
Botox is classified as a medicine, which must be prescribed by a doctor, although it does not have to be injected by a doctor. The injectable fillers for sale in the UK can be bought and sold with no such restriction. Taber said: "Unless we get this sorted out, dermal fillers will be the next disaster."
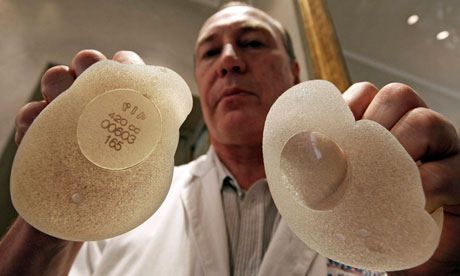
Plastic surgeons said all women in the UK who had had breast implants made by the French company PIP should have them removed. Tim Goodacre, a member of the government-commissioned panel investigating the PIP scandal, and president of the British Association of Plastic, Reconstructive and Aesthetic Surgeons, said: "Given the fact that there is uncertainty and lack of knowledge in this, we really are recommending that all [faulty] implants do come out." Goodacre told the BBC: "Even with a very low rupture rate, we'd want to see most implants removed on a staged basis. If you believe a device is faulty – I think this would be true in your car, or any other object that you buy – you'd want to have that replaced."
French officials shut down the manufacturers in 2010 and supplies were stopped in Britain after it was found to be making prosthetics from cheaper industrial silicone normally used in electronics. The implants were also found to have a higher chance of bursting. The owner is facing criminal prosecution in France.
The scandal deepened in France last month when officials reported eight cases of cancer in women with the implants. Fazal Fatah, president of the British Association of Aesthetic Plastic Surgeons, said that although the implants were not known to cause cancer, women should not be asked to tolerate implants with a high rupture rate, which could cause distressing side-effects.
His members had published evidence as early as 2006 that the implants were substandard, but nobody took any notice, Fatah said. "When the French finally discovered these implants were faulty, we concurred with the French view that because of the poor quality it is advisable to remove them." The industrial-grade silicone does not stay in one piece when the implant ruptures, but spreads around the area, causing lumps and irritation. Fatah said breast cancer was such a common fear that it was beyond belief that women should be put through such anxiety. "To wake up one morning with a tender lump in the breast … These implants are not fit for implanting."
On Saturday the health secretary, Andrew Lansley, announced a review of the risks amid concerns that evidence about potential dangers was unreliable.
French authorities have already recommended women have them removed, but the UK's Medicines and Healthcare Products Regulatory Agency (MHRA) has not followed suit. British officials say their data suggests the risk of rupture is 1% rather than the 3.6% estimated by the French. But the government has been told by a private cosmetic surgery firm that 7% of PIP implants ruptured; this compares with 0% over five years in an ongoing study by Nagor, a British maker using proper medical silicone.
The MHRA will also be conducting an audit of evidence to resolve concerns about the "content and quality of the data that cosmetic surgery providers are sharing with the regulator".
Burnham said the protocol with the industry should include three measures as a minimum. Women who asked for their medical records from cosmetic surgery clinics should get them immediately and with no charge. "It is unacceptable that some are being asked to wait or even pay an admin fee. Second, where there is evidence of a rupture, private providers must arrange for urgent removal at no expense to the individual and with any costs to the NHS reimbursed. Third, all women given PIP implants must be offered an urgent, free consultation with a doctor once the results of the urgent review are known."
Fatah said that hardly any of his own members – just two, of around 230 plastic surgeons who work in the NHS as well as privately – had used implants made by PIP (Poly Implant Prosthese), according to the association's own survey.
The PIP implants were far cheaper than the high-quality alternatives, at £50 for each compared with between £300 and £400 each for those made by Nagor, the only British manufacturer. They were sold by volume, meaning they were bought by the large, competitive cosmetic surgery chains and also by the NHS for use in women who had breast reconstruction after cancer or breast deformities.
PIP implants, just like Nagor's, were CE-marked, which means they were certified as of the appropriate quality standard by the regulator in their home countries. Douglas Black, of Nagor, said inspectors from the MHRA visited its factories in Scotland and England two or three times a year; similar checks appear not to have been successfully made at PIP in France.
The world's leading maker of implants, the US company Allergan, has moved its production from Ireland to Costa Rica in the past couple of years. A spokesman said, however, that inspectors from Europe as well as the US continued to visit.
Fatah said patients had no idea whether they were getting a cut-price or an expensive implant; cosmetic surgery clinics charged a sum that included the cost of the implant, along with the surgeon's fee. "The vast majority of people who had PIP implants weren't told," he said.
The scandal highlighted the urgent need for better regulation of the entire cosmetic surgery industry, Fatah said. He added that he would like people who wanted cosmetic surgery to be told to seek the advice of their GP. "Your doctor knows a number of qualified, registered plastic surgeons in your area, who usually work for the NHS as well. This is outside the NHS, but the GPs do understand that they have a duty of care at least to point you in the right direction."BAAPS says a register of implants would have picked up the problems with PIP products within a year, because surgeons or clinics would have to report a rupture. "BAAPS has asked for the establishment of a new implant register for some time," said Fatah. The main suppliers and the MHRA support the idea, he said, but it would need government backing and start-up funds to get off the ground. The final push is needed from the government, because it is a significant commitment." An earlier attempt failed because it depended on patients voluntarily submitting details of their operation to a register — something many were not happy to do. Fatah said the register must hold details of what happened to each implant, which surgeon performed the operation and at which clinic.
Stephen Dorrell, chair of the all-party select committee on health, said on BBC radio that there may be questions over the regulation of the industry.
"It does seem to be surprising, if it is true, that devices have been inserted into patients without a proper audit trail," he said. But, he added, "in the immediate term the important issue is that there are many women who have had concerns raised about the quality of implants that have been inserted into them. The immediate question s that those concerns need to be addressed and resolved."

